Analytical Tools & Techniques in Hot Melt Extrusion
Case Study on Formulation Development
Hot melt extrusion has been widely used as a processing method for many purposes, including formation of a solid molecular dispersion to increase the bioavailability of poorly soluble drugs1. Analytical tools and techniques can greatly reduce time and improve success rates in development of hot melt extrusion formulations.
In the formulation development stage, analytical characterization of molecular dispersion simplifies the process to compare and contrast different hot melt formulations.
Analytical Techniques for HME Formulation Development
With the advent of smaller extrusion equipment, melt extrusion processing of drug substances can now be performed on the milligram scale. Solid molecular dispersions of nifedipine2, nimodipine3 and itraconazole4 have been successfully produced using melt extrusion technology. Analytical characterization of dispersions prepared by melt extrusion is necessary to assess its physical and chemical properties and performance in the final drug product. Interpretation of the analytical data can be challenging. A step wise approach for characterizing a molecular dispersion simplifies the process to compare and contrast different formulations. Testing a formulation at the next step only occurs if acceptable results are obtained.
Microscopy, thermal analysis, spectroscopy and non-sink dissolution test methods are frequently used to characterize formulation candidates and provide product performance and stability information5. Characterizing the dispersion formulations in 3 steps can reduce evaluation and development time. The first step to evaluate the quality of a molecular dispersion prepared by melt extrusion uses microscopy (Light or Scanning Electron Microscopy) and thermal analysis methods (Differential Scanning Calorimetry). Microscopy is used for a visual assessment of the dispersion to detect the presence of drug crystals on or within the dispersion, and is usually the most sensitive method to identify crystals. Crystals can seed formation of other crystals, and ultimately a reduction in product performance. The presence of a small number of crystals may be due to exceeding the carrying capacity of the polymer. A formulation with a lower drug loading may provide an improved molecular dispersion.
Modulated Differential Scanning Calorimetry (mDSC) is used to qualitatively confirm that the dispersion has a single glass transition temperature (Tg) and identify a value for the Tg, and the lack of a melting point corresponding to the drug substance. It is important to run a physical blend of the formulation as a control. Often, the drug may dissolve into the polymer as the temperature is increased during the test.
The 2nd Step completes the assessment of dispersion quality using methods to determine crystallinity (e.g., x-ray powder diffraction (XRPD) and or Raman spectroscopy) and evaluates performance using a non-sink dissolution test. XRPD or Raman can be used to identify the presence of crystals within the sample. Again, it is important to analyze a physical blend of the formulation. Formulations with low drug loadings may be below the sensitivity of the method to detect crystals. Non-sink dissolution testing evaluates each dispersion formulation for enhancement and sustainability of supersaturation over the crystalline drug form, and can be used to rank-order formulations.
The 3rd Step encompasses tests to evaluate physical and chemical stability of the dispersion, generally by mDSC and High Performance Liquid Chromatography (related substances). DSC is used to analyze how the Tg changes as a function of humidity. This test is used to rank-order formulations based on the value of the Tg at a constant equilibrated humidity, such that the highest Tg formulations would have the best predicted physical stability for miscible mixtures of drug and polymer. The related substance test by HPLC determines, under the processing conditions used to manufacture the dispersions, no chemical degradation of the drug substance occurred.
Formulation Development Case
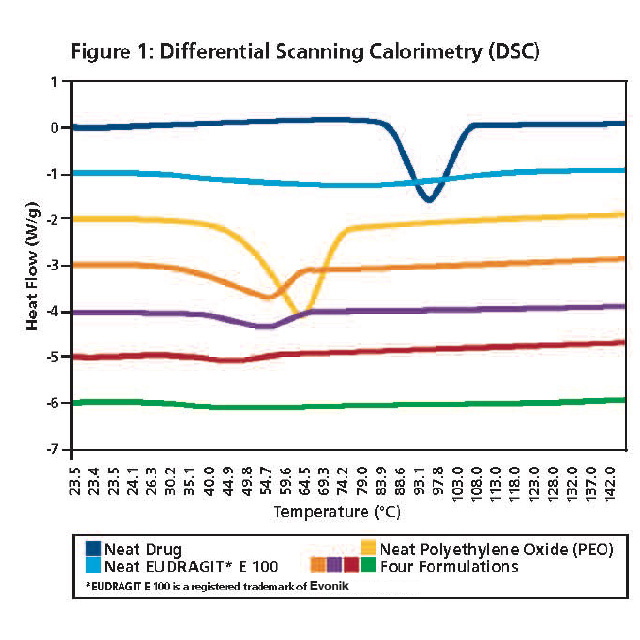
An example of the 3 step analytical characterization approach is presented below. Melt extrusion of a poorly water soluble drug substance was evaluated in 2 different polymers (Eudragit E 100 and Polyethylene Oxide) at 2 different drug loadings. DSC analysis of the neat drug (dark blue), neat Eudragit E 100 (pink), neat Polyethylene Oxide (PEO, yellow) and the 4 formulations (turquoise, purple, red and green) is presented in Figure 1. Melting points associated with the drug substance are absent from the formulations. Melting points corresponding to PEO are present, and depressed, in the two PEO formulations (turquoise and purple) indicating the drug is plasticizing the polymer. A single glass transition was observed in the two Eudragit E formulations (red and green).
Light and Scanning Electron Microscopy was performed on the neat drug (needle shaped), the polymers and four formulations (data not shown). Crystals were absent in the Eudragit E formulations, but crystals were visible in the PEO formulations. The crystals in the PEO formulations were consistent (size and shape) with the polymer, reinforcing the presence of the PEO melting transition observed in the DSC results.
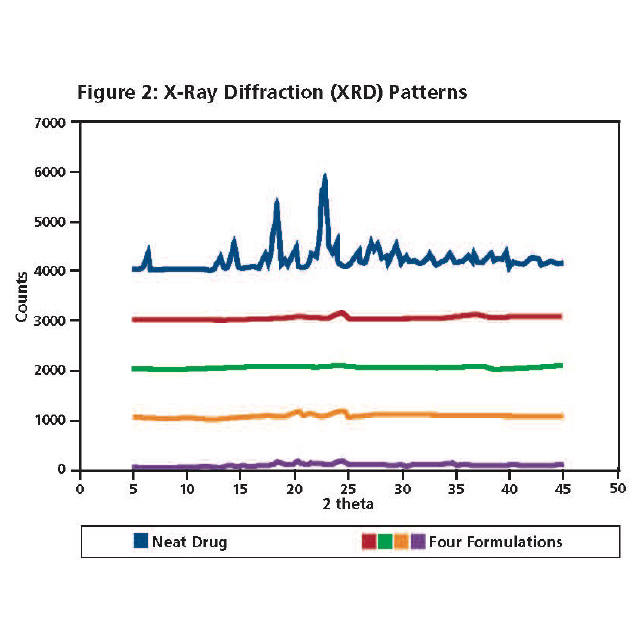
All four formulations were advanced to Step 2 testing. X-Ray Powder Diffraction of the neat drug (dark blue, top) and the four formulations are presented in Figure 2. Physical blends of each formulation (data not shown) indicated the presence of crystalline peaks associated with the drug substance, and peaks associated with PEO in those respective formulations. Crystalline peaks associated with the drug substance are absent in the melt extruded formulations.
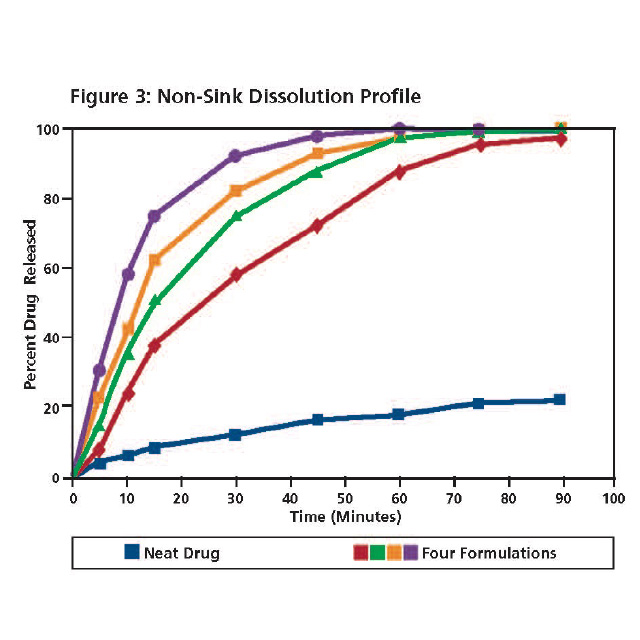
Non-sink dissolution testing of the neat drug (red) and the four formulations (purple, turquoise, green and blue) is presented in Figure 3. All four formulations achieved a super saturation of the drug substance within the 90 minute time frame of the test. Samples were also taken at 4, 8, 24 and 36 hour time points to assess the sustainability of super saturation (data not shown). Three formulations maintained super saturation at the 8 hour time point. All four formulations were unable to sustain super saturation at the 24 hour time point.
Three formulations were advanced to Step 3 testing. HPLC analysis of the molecular dispersion prepared by melt extrusion demonstrated the maintenance of chemical stability. Degradants were not observed in the three formulations. The formulations were also placed into a stability chamber at 40°C and 75% for 4 weeks in open containers. The samples were analyzed by DSC at 1 week, 2 week and 3 week time points (data not shown). The Eudragit E formulations maintained the glass transition temperature observed initially. The PEO formulation adsorbed a significant amount of water and softened, but the presence of a thermal event associated with the drug substance was not observed. A thermal event associated with the boiling point of water was observed.

Conclusion
Melt extrusion processing is technique widely used to form solid molecular dispersions of a drug in a polymer to enhance bioavailability. Extrusion experiments and analytical tests may be performed on a small scale to conserve costly API. A step wise approach to characterizing formulation prototypes using microscopy, thermal analysis, spectroscopy, non-sink dissolution testing and chromatography can be used to rapidly rank order formulations.
FIGURES
Techniques for HME Process Scale-up
Formulation development using hot melt extrusion is generally performed using small, laboratory equipment. Scale up of these developed formulations by achieving similar properties of the dosage forms is always a challenge in pharmaceutical industry. There is limited information available in the literature for scale up of solubility enhanced formulations prepared by melt extrusion processing. Analytical characterization and techniques are critical in the scale up of these melt extruded solid dispersions in order to ensure similar products are produced, specifically in obtaining similar solubility enhancing effect.
When attempting to achieve similar solubility for a formulation on a large extruder to that of the lab scale, the first step is to match process energies between the extruders, both mechanical and thermal. Mechanical energy influences the degree of mixing achieved in the process and thermal energy determines the amount of heat the formulation experiences in the process. Matching energy input of the extruders ensures good mixing without degrading the formulation.
Computer aided process simulation is used to match energies between the small and large extruder. This requires thermodynamic and rheological characterization of the formulation. The simulation provides an initial screw design and process conditions for the larger extruder that provides similar mechanical and thermal energies to the small extruder.
Once initial a screw design and process conditions are established for the large extruder, extrusion trials provide samples that can be analytically characterized and compared to the original samples. Iterative trials to fine tune process conditions may be required to achieve optimal results.
- ¹) MM Crowley, F Zhang, MA Repka, S Thumma, SB Upadhye, SK Battu, J McGinity, C Martin (2007) Pharmaceutical Applications of Hot-Melt Extrusion: Part I. Drug Development and Industrial Pharmacy 33: 909-926
Authors
Tony Listro – Managing Director, Foster Delivery Science
Tony Listro is Vice President, Technology and Site Lead of Sever Pharma Solutions Putnam, CT. Tony is an expert in the areas of polymer materials and polymer processing. He has worked on blending active pharmaceutical ingredients with polymers for various drug delivery applications including oral and implantable dosage forms for more than 15 years. Tony holds both a BS and MS in Plastics Engineering from the University of Massachusetts in Lowell, MA, and an MBA from the University of Massachusetts in Amherst, MA. He holds 2 issued US patents and has authored and/or co-authored 20 publications. Tony is a member of the Society of Plastics Engineers, Controlled Release Society, and AAPS.
Michael M. Crowley, Ph.D.- President of Theridian Technologies, LLC
Dr. Crowley has worked in the field of drug delivery and pharmaceutical research for more than 19 years and has previously been employed in senior management roles with PharmaForm, Monsanto Company, Warner-Jenkinson Company and Mission Pharmacal. Dr. Crowley received his B.S. degree in Chemistry from the University of Missouri at St. Louis, an M.A. in Organic Chemistry from Washington University and a Ph.D. in Pharmaceutics from The University of Texas at Austin where he studied under Professor James McGinity. His research interests include physical pharmacy and pharmaceutical technology focused on novel drug delivery.
Kathrin Nollenberger – Evonik Industries AG
AUTHORS
Tony Listro – Managing Director, Foster Delivery Science
Michael M. Crowley, Ph.D.- President of Theridian Technologies, LLC
Kathrin Nollenberger – Evonik Industries AG
